5# The findings
After a
month of research, I successfully obtained the ideal method set-up for
separating eight amines and the Active Pharmaceutical Ingredient (Ibuprofen) compound. All mass spectrum I obtained
has more than a 50% probability of similarity with the standard, according
to the National Institute of Standards and Technology (NIST) Library software.
1) Difference
in boiling point is the key to enhancing the separation. After identifying each
analyte's boiling point, we will be able to optimise the oven temperature
appropriately.
 |
| Figure 4. Oven Temperature Set-up
in Thermo Xcalibur Gas Chromatography Instrument |
As
shown in the above picture, I started my method setting with a very low
temperature (35°C) to accommodate better separation in my mixture sample.
2) The
inlet temperature is critical and must be set according to the analyte’s
highest boiling point to ensure all the analytes evaporate and turn into gas
phases. Diethanolamine has the highest boiling point (268.8°C); therefore,
I set my inlet temperature to 270°C.
 |
| Figure 5. Split/Splitless Set-Up
in Thermo Xcalibur Gas Chromatography Instrument |
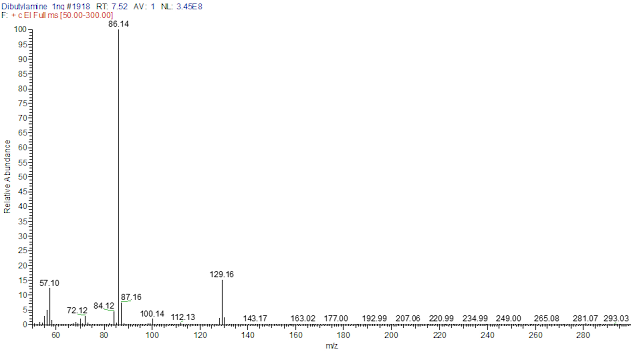
3) Knowing
the compound's structure, formula, exact mass, molecular weight, and m/z is an
excellent way to start! This information will help us set the mass range scans
(in amu units) when using the Mass Spectrometry detector. |
| Figure 6. ISQ General Acquisition Method Setup in Thermo Xcalibur Gas Chromatography Instrument |
Future Study
Another consideration
is that amines in drug products are likely to be in low concentrations. Due to the heavy
matrix of drug samples, extraction or sample clean-up methods must be applied
to reduce matrix interferences, which I’ll do very soon!







Comments
Post a Comment